Abstract
Background: Characterized by the clonal proliferation of bone marrow stem cells and myeloid progenitors, patients with myelofibrosis (MF) have hepatosplenomegaly, abnormal blood counts, bleeding or thrombosis, and suffer debilitating disease-related symptoms. In preclinical models of MF, inhibitors of the Hedgehog (Hh) pathway were shown to inhibit growth and self-renewal of bone marrow stem/progenitor cells that differentiate into cells of the myeloid lineage and to reduce splenic fibrosis. Glasdegib is a potent and selective oral inhibitor of the Hh pathway, acting through the inhibition of Smoothened. Expression of Hh target genes has been shown to be upregulated in granulocytes isolated from MF patients. Here we present recent data from the lead-in cohort of a single arm, open-label phase 1b/2 trial of glasdegib in MF.
Methods: Patients ≥18 years with primary/secondary MF previously-treated with ≥1 Janus Kinase inhibitor (JAKi) were enrolled and received glasdegib 100 mg orally once daily until there was no further clinical benefit. Primary endpoints include adverse events and laboratory abnormalities, as graded by National Cancer Institute Common Terminology Criteria for Adverse Events v 4.03. Secondary endpoints include the proportion of patients with spleen volume reduction ≥35% and the proportion of patients with hematologic improvement. All 21 subjects in the lead-in cohort provided plasma samples for pharmacokinetic (PK) analysis, with 19 subjects providing evaluable glasdegib samples (at steady state) for the purposes of glasdegib PK analysis.
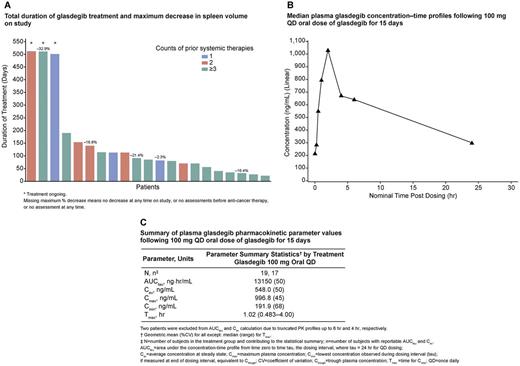
Results: Between October 2014 and October 2015, 21 patients were enrolled in this ongoing study. Mean age was 69 years (range 58-83) and the median duration of treatment with glasdegib was 85 days (range 22-512). Of the 21 subjects, 52% (n=11) were refractory patients who had shown an inadequate response to prior JAKi treatment. A total of five patients achieved a reduction in spleen volume, with a maximum reduction from baseline of 2.3% to 32.9% (Fig A). One patient on study had a positive anemia response, showing improvement from Grade (G) 3 to G2 and became transfusion independent while on treatment. One patient also had an improvement in absolute neutrophil counts (from G2 to G0). Dysgeusia (n=13, 62%), muscle spasms (n=12, 57%), alopecia (n=8, 38%), decreased appetite, fatigue (n=7 each, 33%), increased lipase, decreased weight (n=5 each, 24%), nausea, pyrexia, hyperuricemia, anemia (n=4 each, 19%), asthenia, constipation, cough, dehydration, prolonged electrocardiogram QT, decreased lymphocyte count, myalgia, pain in extremity, thrombocytopenia, and upper respiratory tract infection (n=3 each, 14%) occurred in ≥10% of patients. Despite the high frequency of muscle spasms, there were no significant changes in creatine kinase; only one patient showing a G1 increase compared with baseline. Following administration of 100 mg daily oral dose of glasdegib for 15 days, at steady state, the peak plasma concentrations (Cmax) generally occurred at 1 hour post dose (Fig B). The geometric mean (%CV of geometric means) values for AUCtau and Cmax were 13150 ng.hr/mL (50%) and 996.8 ng/mL (45%), respectively (Fig C). The pre-dose concentrations (Ctrough) following daily dosing were consistent over multiple cycles, with geometric mean Ctrough values of 204.1 ng/mL, 176.1 ng/mL and 189.5 ng/mL respectively, on Cycle 1 day 15, Cycle 2 day 1 and Cycle 3 day 1.
Conclusions: Although some toxicities were observed, these data highlight the potential for glasdegib in patients with primary/secondary MF previously treated with JAKi. Following oral administration of 100 mg oral glasdegib once daily to steady state, peak plasma concentrations generally occurred at 1 hour post dose and glasdegib exposures were consistent over multiple cycles of dosing. Modest clinical activity was seen in some patients.
Gerds: CTI BioPharma: Consultancy; Incyte: Consultancy. Tauchi: Pfizer Inc: Research Funding. Ritchie: Novartis: Consultancy, Research Funding; Incyte: Honoraria; Celgene: Speakers Bureau; Pfizer Inc: Honoraria. Deininger: Gilead: Research Funding; ARIAD: Consultancy; Ariad Pharmaceuticals, Bristol Myers Squibb, CTI BioPharma Corp, Gilead, Incyte, Novartis, Pfizer, Celgene, Blue Print, Galena: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy; Pfizer: Consultancy; BMS: Consultancy, Research Funding; Celgene: Research Funding; Novartis: Consultancy, Research Funding. Jamieson: Johnson and Johnson: Research Funding; GlaxoSmithKline: Research Funding; Celgene: Research Funding; Wintherix: Equity Ownership; Impact Biomedicines: Equity Ownership. Mesa: Novartis: Consultancy; Ariad: Consultancy; Galena: Consultancy; Incyte: Research Funding; Gilead: Research Funding; CTI: Research Funding; Promedior: Research Funding; Celgene: Research Funding. Heaney: Onconova Therapeutics: Research Funding; Novartis: Consultancy. Shaik: Pfizer Inc: Employment. Zhang: Pfizer Inc: Employment. DiRienzo: Pfizer Inc: Employment. Zeremski: Pfizer Inc: Employment. Chan: Pfizer Inc: Employment. Talpaz: Pfizer Inc: Consultancy, Other: Travel, Research Funding; ARIAD: Other: Travel, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal